Pharmaceutical Manufacturing
Improve Quality and Reduce Cost with Real-time Process Monitoring
NIR Sensor Solutions for PAT, QbD and CM Applications
Pharmaceutical manufacturing is demanding. The need to diligently protect patient health and well-being is always primary, but the obligations of USP and EP compliance can push manufacturers into costly and time-consuming processes that complicate the mission. In response to that challenge, scientists and process engineers have been migrating traditional batch-processing of drugs to leaner methods and, increasingly, to continuous or semi-continuous processes. Key to all these trends is PAT – Process Analytical Technology – to increase quality and productivity and gain critical process insights that inform additional innovation.
Batch processing typically depends on frequent laboratory analysis to assure quality at each stage of the manufacturing process. The laboratory analysis of raw materials, intermediates, and finished products, commonly defined as Quality by Testing (QbT), is time-consuming, carries many low value-added steps, and does not always prevent material waste. By contrast, in a Quality by Design (QbD) environment, raw materials are qualified directly in the receiving area; dryers, granulators, and mixers are equipped to continuously monitor moisture content and particle size of intermediates; blend uniformity is verified in real time with no need for systematic thief sampling; and tablet presses monitor the consistency of the powder flow before compression. PAT assures that the final product is as intended, requiring periodic lab analysis only for confirmation and fine-tuning the PAT calibration. Read on to learn how Near Infrared Spectroscopy (NIRS) can improve every phase of solid dose manufacturing and form the cornerstone of a systematic Quality by Design (QbD) implementation.
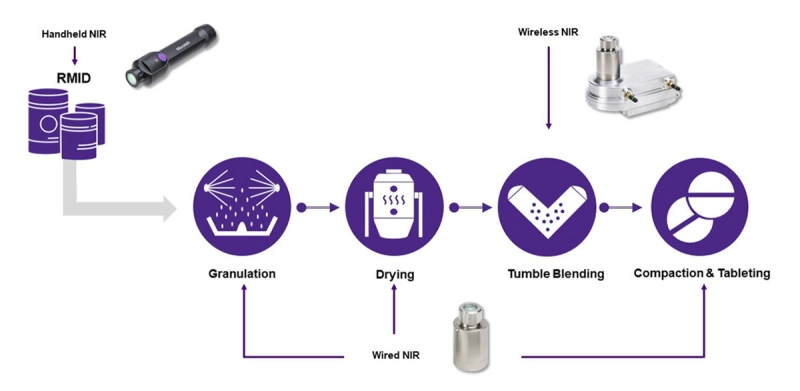
Raw Material Identification (RMID) is the first and fundamental step in a Quality by Design (QbD) process implementation. Ensuring that the material entering the manufacturing process conforms to the desired quality standard is essential to prevent potential failures in process or in the finished product.
International Pharmacopoeias (USP and EP) now require that 100% of incoming material be tested. In a Quality by Testing (QbT) environment, testing a sample from every incoming container puts an unsustainable burden on quality control laboratories. In response, instrument makers like VIAVI have developed a new generation of miniature, fit-for-purpose instruments that can be used to qualify incoming material at the point of action, dramatically reducing the workload on the analytical lab and, in the process, reducing cycle time and floor space requirements.
The MicroNIR™ OnSite-W wireless, handheld spectrometer is an ideal solution for RMID. Its rugged design handles the rigors of loading-dock use. With a long-lived battery that can run days or weeks without charging, its compact, lightweight form and one-button operation maximize productivity and minimize operator fatigue. Read the White Paper Here
Once incoming raw materials are identified and qualified, they are ready to proceed to the next stage of production, which is typically granulation and drying. These processes can also be equipped with NIR spectrometers to monitor the evolution of a process and control its end point.
Fluid bed dryers, granulators and high-shear mixers commonly provide readouts of air and product temperature, air humidity, and torque. These indicators indirectly reflect the progress of an ongoing process, but do not directly measure moisture content and particle size, which are typically critical-to-quality (CtQ) parameters. NIR spectroscopy is very sensitive to both moisture and particle size and can provide direct measures, so integrating NIR spectrometers in FBGDs and HSMs opens a clear window on the dynamics of dry mixing and primary and massing granulation. The insight gained allows the process engineer to understand process variables and variability, identify and define end points, increase process consistency, and minimize process time.
The MicroNIR PAT-U Spectrometer is designed specifically for monitoring processes in fixed equipment. With a single USB cable to provide power and data, the compact, rugged, stainless steel PAT-U is equally at home looking through a window into a process chamber or mounting inside it. Optional mounting flanges allow the PAT-U to readily connect to a wide variety of process equipment, and optional software connects the PAT-U directly to OPC process controllers.
Once raw materials and intermediates are prepared and qualified, they must be blended uniformly to ensure that every unit dose contains an identical ratio of constituents. Blenders take various forms, but one of the primary methods is tumble blending. Tumble blenders present a unique challenge for real-time monitoring since it is not possible to connect a cabled instrument to a piece of rotating machinery. The MicroNIR PAT-W is a unique, lightweight, wireless instrument that can be readily attached to almost any size tumble blender to bring the power of NIR spectroscopy to real-time blend monitoring.
Monitoring blending can be a uniquely simple process. MicroNIR Pro software, included with MicroNIR instruments, contains multiple methods of monitoring the progress of a blender. One such method, Moving Block Standard Deviation or MBSD, is an “unsupervised” method, meaning it requires no prior chemometric modeling to determine the end point of the blending process. It is easy to implement and use. By measuring the NIR reflectance spectrum of the blend on each rotation of the vessel and calculating the standard deviation of spectral variation in time, one gets a direct measure of blend uniformity. When the standard deviation drops below a predetermined threshold, the insignificant difference between successive samples indicates that the blend is uniform. MicroNIR Pro software includes multiple unsupervised and supervised methods for monitoring blend uniformity.
Tableting is one of the final steps in pharmaceutical, nutraceutical and other solid-forms manufacturing. Earlier-stage NIR monitoring of moisture, particle size, and blend uniformity is essential to avoid out-of-specification intermediate mixtures, but problems may still arise at the very last – tableting – step. Sticking and (de)lamination, typical tableting issues, can be caused by fines, trapped air, incorrect moisture content in the powder blend, and many other process deviations. Although physical defects are easy to detect, often by visual inspection of the finished tablets, identifying the source of the problem requires laboratory analyses, after which corrective action can only be taken on future production. The defective batch must be scrapped, which results in material waste and lost time. In absence of tablet physical defects, out-of-specification tablets would be detected only after laboratory analysis and may prompt corrective actions, including product recalls. The implementation of NIR sensing in a tablet press enables the detection of anomalies in the formulation before the compression stage and allows real-time root cause analysis of the problem and immediate corrective action.
The MicroNIR PAT-U and PAT-W instruments make excellent tableting process monitors when fitted on the press chute or feed frame. Download our Application Note for more insight on monitoring tableting processes.
Let Us Help
We’re here to help you get ahead.
